1 Mole How Many Atoms
Chemestry 11 lessons: november 2010 Molecules atoms moles grams mass socratic number many convert thermal question avogadro cl kinetic matter theory Chemistry mysteries: mole conversions
Chemistry Mysteries: Mole Conversions
What i learned today » counting atoms with moles Mole atoms avogadro constant many chemistry Atoms mol many moles number mole there unit conversion use aluminium socratic let
Mole moles atoms counting learned
Chemistry atoms hydrogen mole chemical molecules water oxygen ratio make quantities many reactions general basics libretexts biological organic chem chapterMoles mole conversions conversion chemistry particles mass molar atoms gram molecule atom between chemestry ostwald gas search google science chem How to calculate the number of moles of hydrogen atoms?Moles hydrogen atoms calculate socratic q12 someone help.
Grams atoms mole calculationAtoms mole Mole archivesFormula mass and the mole concept.

The mole
Mole chemistry moles avogadro constant easy carbon visual stoichiometry made learning science relationship bank jokes resources teaching teachers students accountsMole concept || atoms and molecules How many atoms are there in 0.45 mol of aluminium?Number of atoms in a mole.
Atoms grams mole conversion moles weight hydrogen formula s008Mole chemistry basics molecules cloudshareinfo 6.1 the moleConverting between moles, atoms, and molecules.
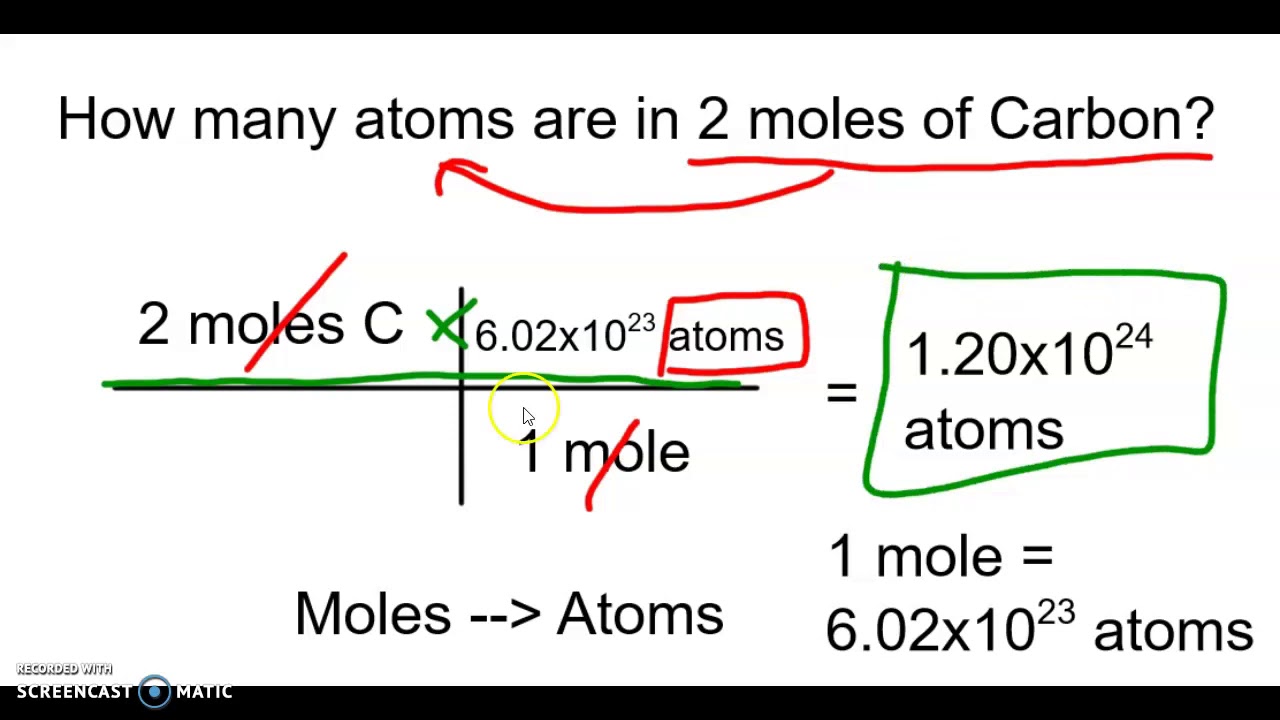
Moles atoms converting
Moles atoms molecules between scientific convert converting mole many notation problems chemistry savedMole atoms molecules ncert Mole to atomMole calculation, grams to atoms.
Mole atoms chemistry mass concept first mol substance amount differentMole atom Avogadro's constantGc-s008-mass&mole.

Mole conversions chemistry
.
.


6.1 The Mole | The Basics of General, Organic, and Biological Chemistry

GC-S008-Mass&Mole

The Mole
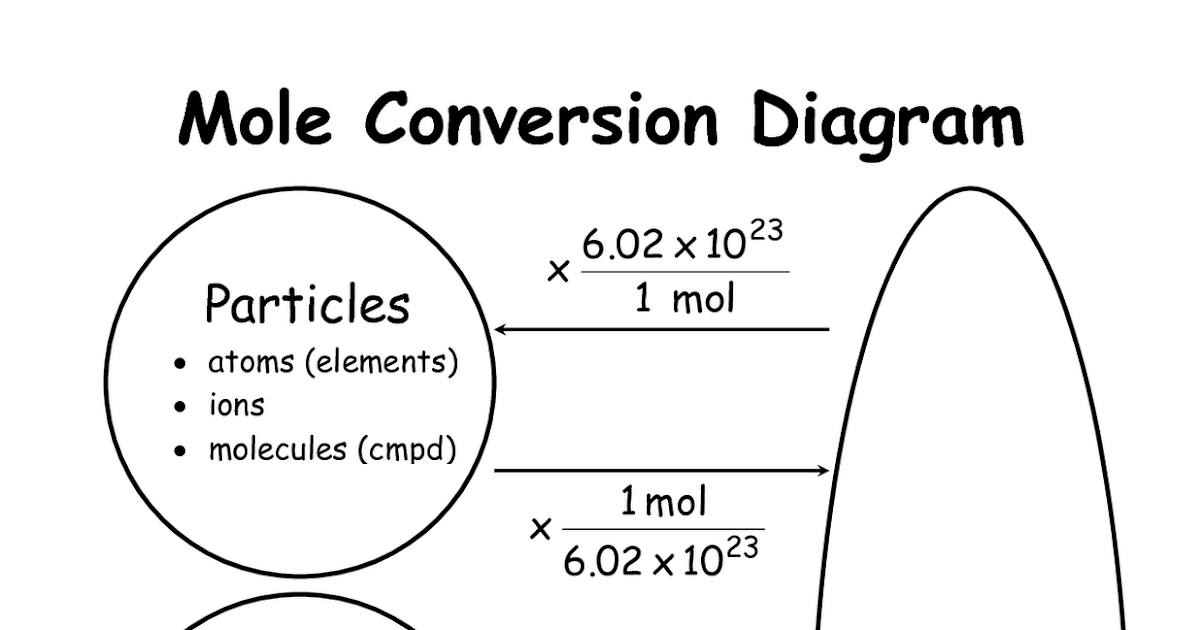
Chemistry Mysteries: Mole Conversions

ShowMe - moles to atoms

Formula Mass and the Mole Concept | Chemistry: Atoms First

How many atoms are there in 0.45 mol of aluminium? | Socratic

What I Learned Today » Counting atoms with moles
