How Many Electrons Are In Aluminum
Aluminium configuration electron al element periodic table elements Aluminum argon shells electrons electron shell orbital chem4kids elements element symbol find there bonding Neutrons protons bohr atom electrons socratic clipart electron nucleus
How many protons, neutrons, and electrons are present in an atom of
How many protons, neutrons, and electrons are present in an atom of Question #da2f7 Solved compute the number of electrons that each aluminum
Solved aluminum electrons compute number transcribed problem text been show has
Chem4kids.com: aluminum: orbital and bonding infoAluminium al (element 13) of periodic table Atom structure 3d aluminum over render electrons protons background isolated white alamy stock neutron spheres represented red cartSulfur silicon magnesium aluminum phosphorus electron orbital configuration shells sulphur energy orbits electrons atom chem4kids many bonding levels valence find.
Electrons atom nucleus socratic orbit outermostElectron potassium silicon configuration sodium aluminium aluminum bohr diagram structure model atomic number magnesium chlorine table configurations element sulphur lewis Electrons valence electron nucleus spinning orbitsChem4kids.com: aluminum: orbital and bonding info.

Neutron electron proton teachoo nucleus examples
3d render of atom structure of aluminum isolated over white backgroundBohr aluminum model silicon electrons elements element diagram atomic structure al si number chemical atom energy level electron table block Electron arrangementsChemical elements.com.
Aluminum: aluminum electrons .
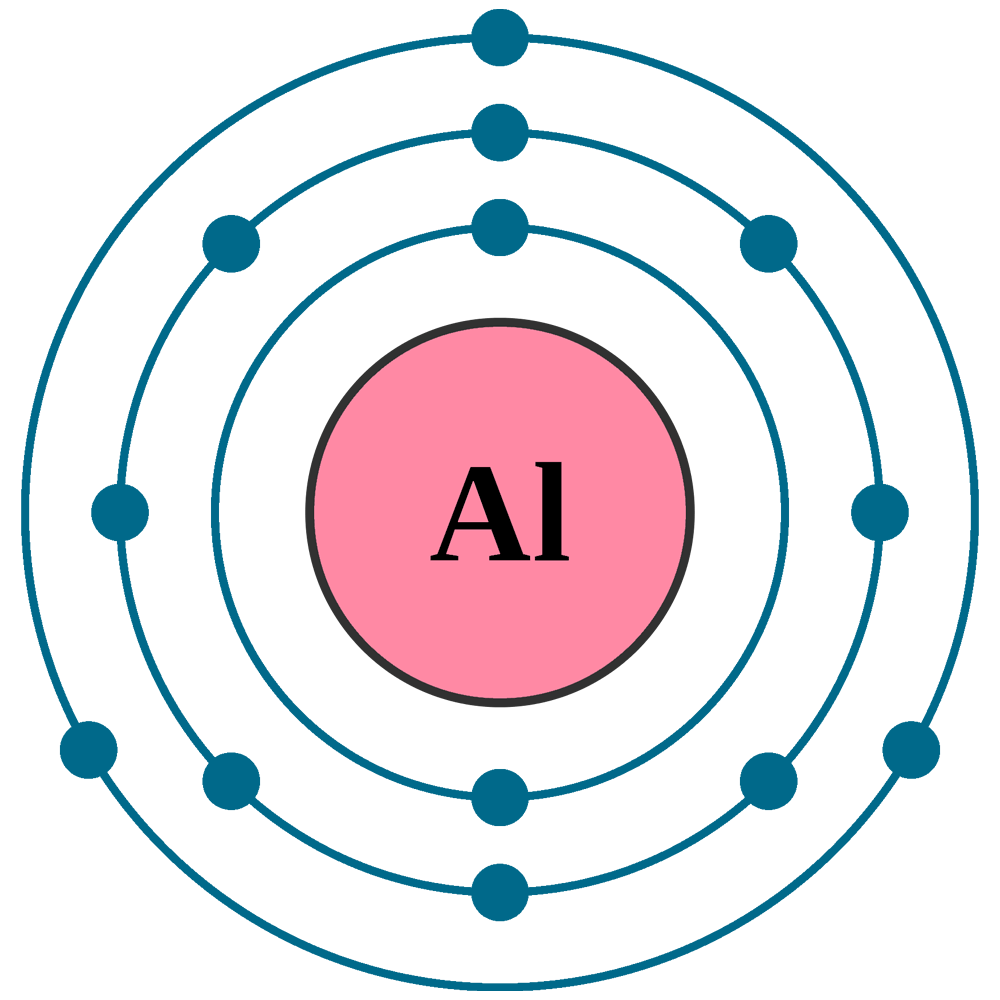

Chem4Kids.com: Aluminum: Orbital and Bonding Info

How many protons, neutrons, and electrons are present in an atom of

Aluminum: Aluminum Electrons

Chem4Kids.com: Aluminum: Orbital and Bonding Info

Chemical Elements.com - Aluminum (Al)

Solved Compute the number of electrons that each aluminum | Chegg.com

3d render of atom structure of aluminum isolated over white background

Electron arrangements

Neutron - Discovery, Difference and more - Teachoo - Concepts
