How Many Neutrons Are In Tin-120
How many protons are in an atom of tin? how many electrons are in an Neutrons tin atom protons Solved an atom of tin has 50 protons, and (typically) 70,
How many protons are in an atom of tin? How many electrons are in an
Silver atomic structure technetium rhodium cadmium neutrons carlos periodic table science photograph c018 chemical ag sciencephoto stock Configuration sn electron elemental percent 10 interesting iodine facts
Facts about tin
Iodine atom facts interestingNeutrons protons An atom of tin has 50 protons, and (typically) 70,How many neutrons does scandium have?||number of neutrons in scandium.
How many protons are in an atom of tin? how many electrons are in anBarcode envelope banded answer Silver periodic table neutronsNeutrons protons many electrons neon chlorine potassium periodic calcium scandium quora isotope chemistry.

Tin-120, tin-120 isotope, enriched tin-120, tin-120 metal
.
.


Silver Periodic Table Neutrons | Review Home Decor

Facts About Tin | Live Science

An atom of tin has 50 protons, and (typically) 70, | Chegg.com
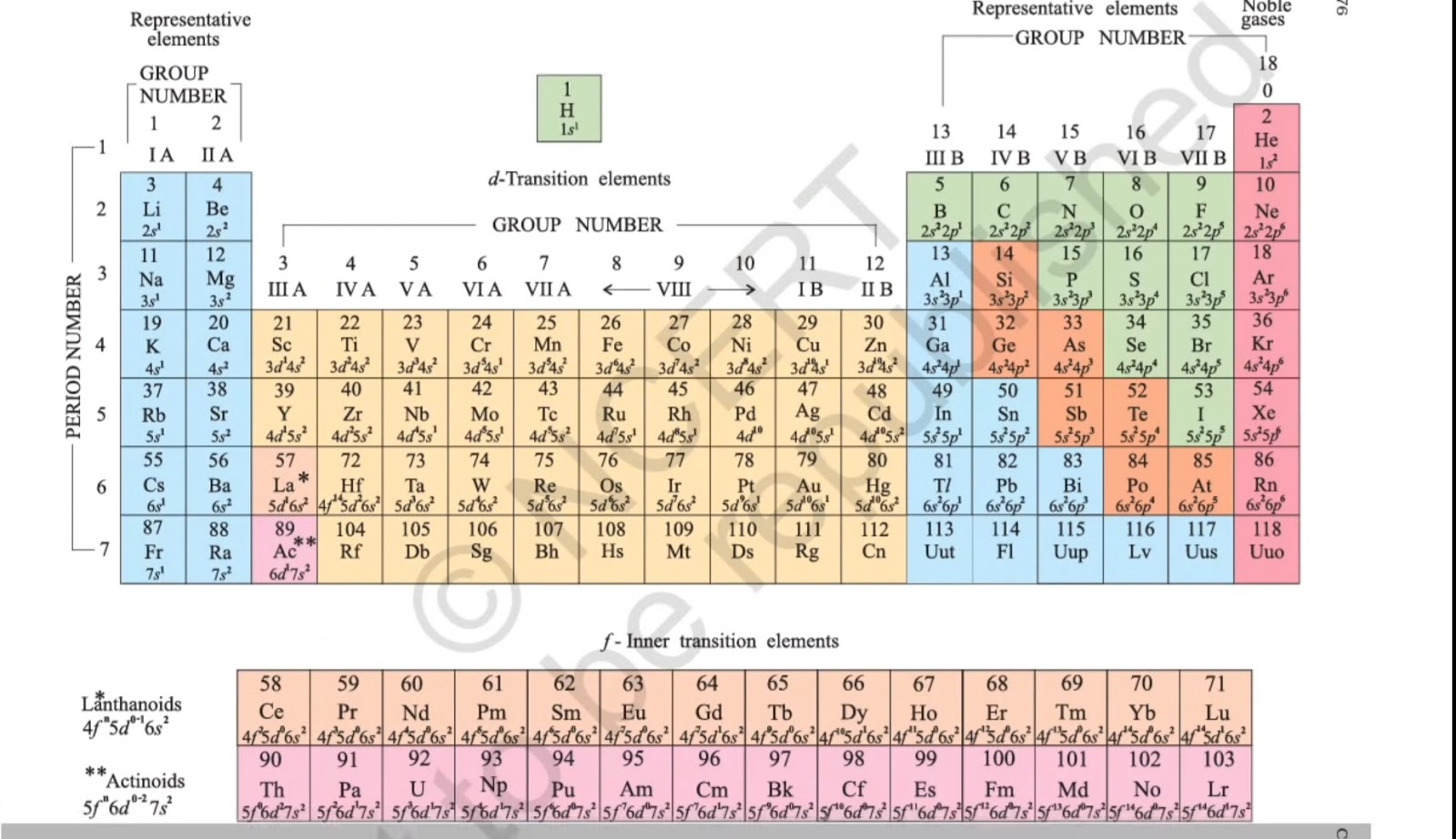
How Many Neutrons Does Scandium Have?||Number of Neutrons in Scandium

10 Interesting Iodine Facts | My Interesting Facts

How many protons are in an atom of tin? How many electrons are in an

How many protons are in an atom of tin? How many electrons are in an
